Categories
Latest blog
Basic principles of XRF analyzer
Mar 29 , 2022Basic principles of XRF analyzer
X-ray is a special device used by German physicist Roentgen in the study of thin gas discharge experiments. This device emits electrons, installs a metal anode at the opposite position of the cathode, and applies a high voltage between the anode and the anode. When the container is filled with the gas is thin, a discharge phenomenon can be observed in the gas. The unknown rays found in the test are named X-rays by Roentgen. X-ray is defined as the electromagnetic radiation emitted when electrons accelerate near the nucleus or when transitions occur between the inner and outer electron energy levels of the nucleus. XRF analyzer is secondary X-rays generated when primary-level X-rays are irradiated to the sample to be measured. The incident X-rays have a relatively large energy, which makes it possible to bombard the electrons located in the inner layer of the element atom. The XRF analyzer has a wavelength between 0.01-10 nm and an energy between 124KeV-0.124KeV. The wavelength range of XRF analyzer used in elemental analysis is between 0.01-11 nanometers, and the energy is 0.111-0.124KeV.
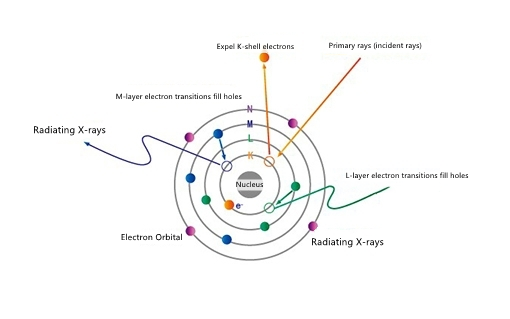
When X-rays excite the characteristic X-rays of the sample, the incident electromagnetic radiation energy must be greater than a certain value to cause the inner electron excited state to form holes and cause electron transitions. This value is the absorption limit, which is equivalent to the inner layer. The work function of an electron. If the energy of incident electromagnetic radiation is below the absorption limit, the electrons in the inner layer of the atom cannot be excited under any circumstances and characteristic X-rays are generated. X-ray excitation.
X-ray excitation. Elements are composed of atoms, and the chemical properties of each atom are exactly the same. Atoms are composed of nucleus and extranuclear electrons. The extranuclear electrons are distributed according to the layered law, thereby forming the electron shell structure of the atom. It is divided into multiple layers from the inside to the outside, and each shell layer can hold a maximum of 2n2 electrons. This is the Pauli exclusion principle. The K layer is the shell layer with the lowest energy level, by the L layer and M layer. The size of the stage is equal to the shell's electronic binding energy. Under normal conditions, high-level electrons fill the low-level electrons to keep the atoms in a stable state. When X-rays and high-energy particle beams are irradiated, the high-energy particles and photons collide with the sample atoms, expelling the inner electrons of the atom. And the hole is formed at its position, so the atom is in an excited state. The lifetime of this excited ion is extremely short. When the outer electrons transition to the inner hole, the extra energy will be released in the form of X-rays and outside, The layer generates new holes and new X-ray emissions, thereby generating a series of characteristic X-rays. If you want to obtain the characteristic X-rays of an element, you need to excite the inner electrons of the element atoms, so that the inner electrons get a certain energy, which can get rid of the nucleus of the atom, so that electron holes are formed in the inner orbits, which are filled by higher-level electrons. This hole will emit characteristic X-rays with a certain energy. This process is the excitation of X-rays, which is the basic principle of X-ray fluorescence.